- Research
- Open access
- Published:
The role of colchicine in the management of COVID-19: a Meta-analysis
BMC Pulmonary Medicine volume 24, Article number: 190 (2024)
Abstract
Background
The Coronavirus disease 2019 (COVID-19) pandemic has robustly affected the global healthcare and economic systems and it was caused by coronavirus-2 (SARS-CoV-2). The clinical presentation of the disease ranges from a flu-like illness to severe pneumonia and death. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million. Colchicine is an alkaloid drug that is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine inhibits the production of superoxide and the release of interleukins that stimulate the inflammatory cascade. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) that are related to the fibrosis. Moreover, colchicine has been used to traet viral myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. Additionally, colchicine is considered safe and affordable with wide availability.
Objective
The aim of the current study was to assess the evidence of colchicine effectiveness in COVID-19 treatment.
Methods
A comprehensive review of the literature was done till May 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only 8 clinical trials and cohort studies fulfilling the inclusion criteria were included for further steps of data collection, analysis, and reporting.
Results
This meta-analysis involved 16,488 patients; 8146 patients in the treatment group and 8342 patients in the control group. The results showed that colchicine resulted in a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care (RR 0.35, 95%CI: 0.15–0.79). Colchicine resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95%CI 0.02–0.27, P = 0.000024). However, colchicine had no significant effect on the following outcomes among COVID-19 patients: the need for hospitalization, ICU admission, artificial ventilation, and hospital discharge rate. Among the PCR confirmed COVID-19 patients, colchicine decreased the hospitalization rate (RR 0.75, 95%CI 0.57–0.99, P = 0.042). However, colchicine had no effect on mortality and the need for mechanical ventilation among this subgroup.
Conclusion
Colchicine caused a significant clinical improvement among COVID-19 patients as compared with the standard care or placebo, in terms of the need for O2, and mortality. This beneficial effect could play a role in the management of COVID-19 especially severe cases to decrease need for oxygen and to decrease mortality among these patients.
Introduction
The Coronavirus disease 2019 (COVID-19) that was caused by coronavirus − 2 (SARS-CoV-2) has significantly impacted the healthcare and economic systems worldwide. The disease first began in Wuhan, China at the end of 2019. Then, it spread worldwide and became a pandemic. The clinical picture of the disease ranges from a flu-like illness to a massive inflammatory response and death [1]. In 2002 and 2003, there were outbreaks of severe respiratory distress syndrome in China. They occurred by SARS-CoV, another member of the coronavirus family. In 2012, another outbreak was documented in the Middle East and was caused by Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. The current coronavirus is characterized by higher infectivity and geographical spread in comparison with both SARS and MERS. Therefore, COVID-19 was considered a significant global health threat that required robust efforts to minimize the burden of this pandemic [3].
The World Health Organization (WHO) announced that COVID-19 is a pandemic on 11 March 2020 [4]. Since then, the number of COVID-19 patients significantly increased. Till September 2022, the cumulative number of cases exceeded 600 million worldwide and deaths were more than 6 million [5].
The clinical manifestations of COVID-19 encompass symptoms such as fever, cough, dyspnea, malaise, or anosmia or ageusia, which can aid in early detection of the disease [6]. The primary mode of COVID-19 transmission is predominantly through exposure to infectious respiratory droplets from close contact with either symptomatic patients or asymptomatic carriers, as well as through aerosol particles that can remain suspended in the air for extended periods [7]. Additionally, indirect transmission through contaminated fomites, fecal excretion, environmental contamination, and fluid pollution has been documented, with viral viability reaching up to 72 hours after infecting surfaces [7, 8].
SARS-CoV-2 is a beta coronavirus that is a positive-stranded enveloped RNA virus. Similar to SARS-CoV and MERS-CoV, it is found in domestic and farm animals [9, 10]. The SARS-CoV-2 is characterized by spike proteins called S proteins. These proteins facilitate the viral infection through binding the S proteins and the angiotensin-converting enzyme 2 receptors (ACE2). These receptors are found in many tissues such as pneumocytes, enterocytes, renal cells, and endothelial cells [11]. SARS-CoV-2 causes marked dysfunction of the epithelial barrier and the endothelial cells of the pulmonary capillaries which triggers the migration and accumulation of inflammatory cells. This initiates the inflammatory cascade by both innate and cell-mediated immunity which significantly influences the alveolar-capillary oxygen transmission and the oxygen diffusion capacity [12].
In severe cases of COVID-19, fulminant inflammation, stimulation of the coagulation pathways, and consumption of the clotting factors occur in the form of a “cytokine storm”. This happens under the effect of many inflammatory mediators including interleukins, tumor necrosis factor-α (TNF-α), and interferon (IFN-γ). In addition, vasodilators such as bradykinin increase vascular permeability and result in pulmonary edema [13].
These mechanisms of cell damage represent a target for already existing medications that modulate the immune response. Based on its anti-inflammatory effects, colchicine has gained attention to be utilized in the management of COVID-19 patients. Colchicine is an alkaloid drug that is formed from a plant called “Colchicum autumnale”, also named “autumn crocus”. Colchicine is used in many autoinflammatory conditions e.g., gout, familial Mediterranean fever, and Behçet’s syndrome. Colchicine has an anti-inflammatory effect that is mediated through its binding to the tubulins and inhibiting the polymerization of microtubules. Microtubules are a key component of the cytoskeleton and are composed of tubulin heterodimers. These structures are important in different cellular functions including intracellular trafficking, cell shape, cell migration, and division [14]..
Colchicine inhibits the production of superoxide and the release of interleukin 1β and IL-6. Colchicine also prevents the inflammatory cascade by decreasing the production of inflammasomes that stimulate caspase-1 activation and release of interleukins such as interlukin1β and interleukin IL18 [15, 16]. Colchicine decreases the differentiation of myofibroblast and the release of fibrotic mediators including transforming growth factor (TGF-β1) [17, 18]. Moreover, colchicine has been used in cardiac conditions caused by a viral infection like myocarditis caused by CMV or EBV, interstitial pneumonia, and pericarditis resulting from influenza B infection. These different mechanisms greatly decrease the inflammatory response that represents a cornerstone in the pathophysiologic process of COVID-19. Besides the aforementioned effects of colchicine, its usage is considered safe and affordable with wide availability [19].
The ongoing impact of COVID-19 on all life aspects, the scarcity of effective treatments and the emergence of new virus variants resulted in the urgent need to repurpose the already existing drugs and to invent new therapeutic agents. This raised concerns about the effectiveness of colchicine in COVID-19 treatment and the possibility of providing an improvement in the clinical course of the disease.
The aim of the current study was to evaluate the efficacy of colchicine on different clinical outcomes including mortality, duration of COVID-19 illness till recovery, need for hospitalization, need for O2 therapy, need for ICU admission, and need for artificial ventilation.
Methodology
Criteria for considering studies for this meta-analysis
Types of studies
The review was restricted to Clinical Trials and Cohort Studies, which investigated the Colchicine administration in COVID-19 patients, versus standard treatment/placebo.
Types of participants
Participants were adult patients with the diagnosis of COVID-19. Patients were considered to have a definite diagnosis of COVID-19 if they were laboratory-confirmed using reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. All healthcare settings (community/primary care, hospital outpatient, or long-stay institutional) were considered eligible.
Types of interventions
Clinical trials and Cohort Studies were included. Colchicine was administered in COVID-19 patients, versus standard treatment/placebo.
Types of outcome measures
At least one of these outcomes was considered; Mortality, Duration of COVID-19 illness till recovery, Need for hospitalization, Need for O2 therapy, Need for ICU admission, and Need for artificial ventilation.
Inclusion criteria
(i) Cohort studies. (ii) Randomized and non-randomized clinical trials. Studies conducted on adult human subjects. (iii) Studies conducted on patients diagnosed with COVID-19 confirmed with positive reverse transcription polymerase chain reaction (RT-PCR) and/or high-resolution CT chest with CO-RADS 4 or 5. (iv) Studies conducted in all healthcare settings (community/ primary care, hospital outpatient or long-stay institutional). Studies published in Arabic, English, French or Spanish languages.
Exclusion criteria
Review, opinion studies, Case series, Studies conducted on animals.
Search strategy for identification of studies
Published studies and abstracts on the role of colchicine in the management of COVID-19 were identified through a comprehensive search of electronic databases that included PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect (www.sciencedirect.com), Scirus (www.scirus.com/srsapp), ISI Web of Knowledge (http://www.isiwebofknowledge.com), Google Scholar (http://scholar.google.com) and CENTRAL (Cochrane Central Register of Controlled Trials (http://0-www-mrw-interscience-wiley-com.brum.beds.ac.uk/cochrane/cochrane_clcentral_articles_fs.htm), using a combination of the following keywords: “Colchicine, COVID-19, Clinical Trail, Cohort Study”.
Methods of the meta-analysis
Locating and selecting studies
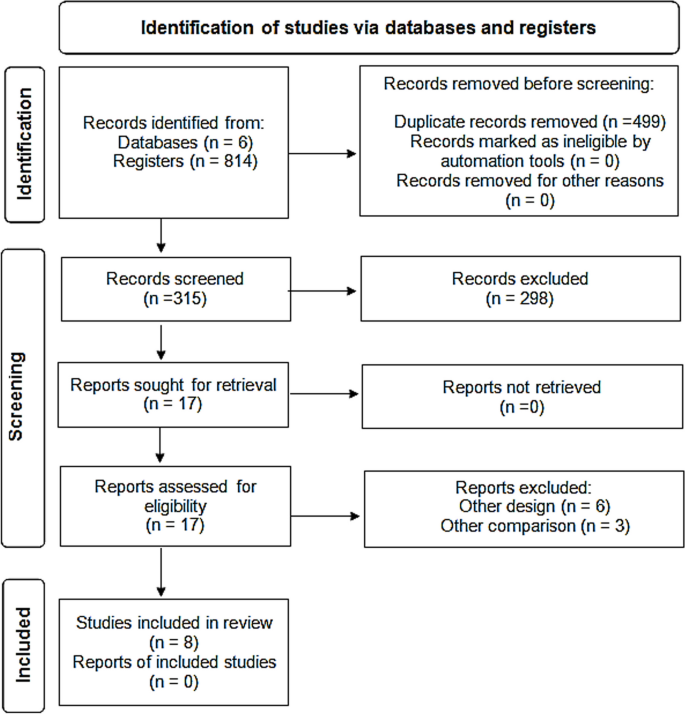
Abstracts of articles identified using the search strategy above mentioned were viewed, and articles that appeared to fulfil the inclusion criteria were retrieved in full. Data on at least one of the outcome measures was included in the study. Each article identified was reviewed and categorized into one of the following groups: Included: Randomized and non-randomized clinical trials, and Cohort studies that met the described inclusion criteria and those where it was impossible to tell from the abstract, title or MESH headings. Excluded: review, opinion studies, case series, and studies conducted on animals. When there was a doubt, a second reviewer (MFA) assessed the article, and a consensus was reached. The literature was reviewed till May 31, 2022 and yielded 814 articles after ranking the articles according to authors and year of publication. Only articles fulfilling the inclusion criteria were included (total 8 articles) for further steps of data collection, analysis, and reporting. The studies that met our inclusion criteria were Deftereos et al., Tardif et al., RECOVERY Collaborative Group, Lopes et al., Sandhu et al., Mareev et al., Brunetti et al. and Scarsi et al. [20,21,22,23,24,25,26,27]. All were in English and there were no available studies published in Arabic, French or Spanish language.
Data extraction
A copy of each identified paper was obtained, and relevant data was abstracted by the first reviewer for a quantitative overview. We extracted the following study data from full-text articles: first author name, year of publication, study design, study location, eligibility criteria, sample size, age, sex, description of intervention and control groups, primary and secondary outcomes. In case of discrepancies or when the information presented in a study was unclear, abstraction by a second reviewer (MFA) was sought to resolve the discrepancy.
Statistical considerations
Data were abstracted from every study in the form of a risk estimate and its 95% confidence interval. When a risk estimate and its 95% confidence interval were not available from the article, we calculated unadjusted values from the published data of the article, using the Epi Info 6 computer program version 6.04d.
Pooled estimates of relative risks were obtained by weighing each study by the inverse variance of the effect measure on a logarithmic scale. This approach to pool the results assumed that the study populations being compared were similar and hence corresponded to a fixed effect analysis. The validity of pooling the relative risks was tested (test of homogeneity) using chi square test.
A violation of this test suggested that the studies being pooled differed from one another. In the presence of significant heterogeneity of the effect measure among studies being compared, we performed a random effect analysis that was based on the method described by DerSimonian and Laird. The random effect analysis accounted for the interstudy variation. Because the test of homogeneity had low power, we reported the figures of the random effect analysis even with the absence of significant heterogeneity.
All statistical analyses for pooling the studies were performed on the MetaXL Software.
Results
In 6 databases, we identified 814 articles; 499 duplicates were removed. Out of the remaining 315 abstracts, we excluded 298 after screening. Thus, 17 full-text studies were assessed for eligibility and 9 were excluded. Finally, eight studies were included for further qualitative and quantitative analyses (Fig. 1).
Characteristics of the included studies
Two studies were cohort (Brunetti et al. and Scarsi et al.) while the other studies were four randomized controlled clinical trials (Deftereos et al., RECOVERY Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized controlled clinical trials (Mareev et al., and Sandhu et al.).
Two studies were multicentre clinical trials (RECOVERY Collaborative Group, and Tardif et al.). The other six studies were conducted in Greece (Deftereos et al.), Brazil (Lopes et al.), the USA (Brunetti et al. and Sandhu et al.), Russia (Mareev et al.), and Italy (Scarsi et al.) [22,23,24,25,26,27,28,29].
The studies included both hospitalized and non-hospitalized COVID-19 patients, who were diagnosed either clinically or by laboratory diagnosis with PCR–RT testing and CT chest imaging (Table 1).
Mortality
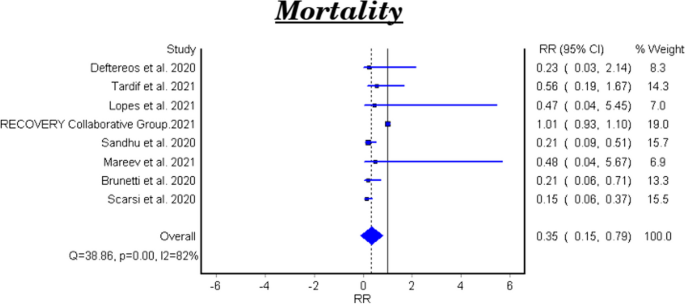
Table 2 and Fig. 2 showed that the meta-analysis of all included studies showed a significant difference in mortality between the treatment group with colchicine and the control group (RR 0.35, 95% CI: 0.15–0.79). There is significant heterogeneity among the studies (Homogeneity Test X2: 42.219, P-value < 0.000).
The meta-analytical result of the six clinical trials was insignificant between the treatment and control groups (RR 0.48, 95% CI 0.22–1.07). There is significant heterogeneity among the studies (Homogeneity Test X2: 11.562, P-value: 0.000). The meta-analytical result of the two cohort studies was significant between the treatment and control groups (RR 0.17, 95%CI 0.08–0.35).
Duration of COVID-19 illness till recovery
Table 3 shows the efficacy of colchicine on the duration of COVID-19 illness till recovery. Lopes et al. reported that the median duration of COVID-19 illness in the treatment group with colchicine was 7 days vs 9 days in the control group (P-value =0.003) [25]. While Sandhu et al., and Mareev et al., demonstrated that colchicine had no significant effect on the illness duration [26, 27]. (Table 3).
Need for hospitalization
Tardif et al., reported that colchicine did not show a significant effect on the COVID-19 patients’ need for hospitalization RR 0.79, 95% CI 0.60–1.03, P-value =0.081) [23].
Need for O2 therapy
Lopes et al., demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 (RR 0.07, 95% CI 0.02–0.27, P = 0.000024) [25].
Need for ICU admission
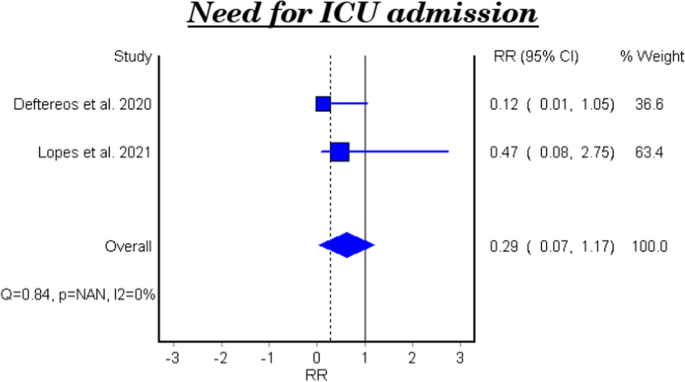
Table 4 and Fig. 3 show the efficacy of colchicine on need for ICU admission in patients with COVID-19. The meta-analytical result did not show a significant effect (RR 0.29, 95% CI: 0.07–1.17).
Need for artificial ventilation
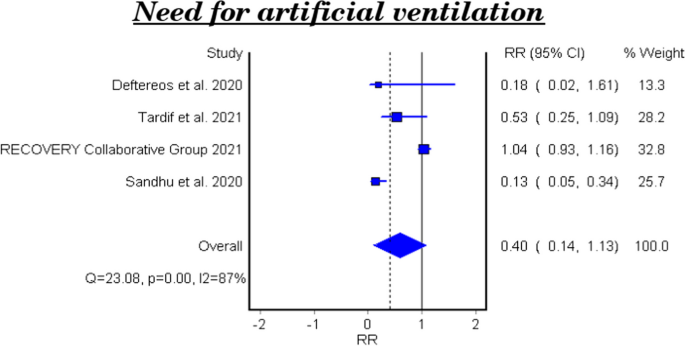
Table 5 and Fig. 4 show the efficacy of colchicine on need for artificial ventilation in patients with COVID-19. The meta-analysis of four studies demonstrated that colchicine has no significant effect on the need for artificial ventilation (RR 0.40, 95% CI 0.14–1.13). There is significant heterogeneity among the studies (Homogeneity Test X2: 18.417, P-value: 0.000).
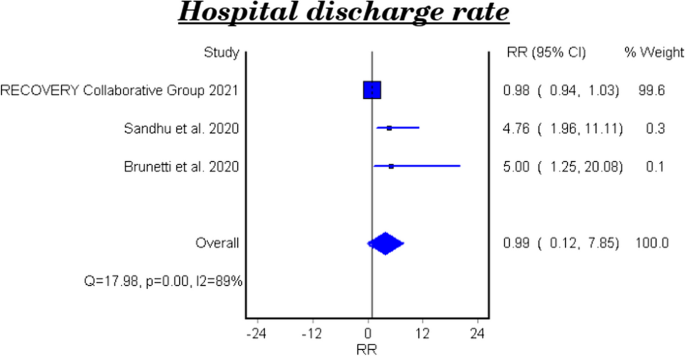
Hospital discharge rate
Table 6 and Fig. 5 show the efficacy of colchicine on hospital discharge rate in patients with COVID-19. The meta-analytical result of the three studies demonstrated that colchicine did not show a significant effect on the hospital discharge rate (RR 0.99, 95%CI 0.12–7.85).
The effect of colchicine on the hospital discharge rate in the clinical trials was not significant (RR 0.98, 95%CI 0.12–8.02), while a cohort study reported that colchicine showed a significant effect on the hospital discharge rate (RR 5.0, 95%CI 1.25–20.08, P-value 0.023) [28].
Subgroup analysis among PCR confirmed COVID-19 patients
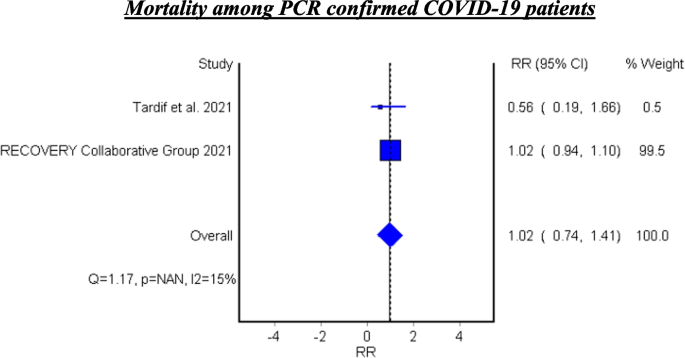
Mortality among PCR confirmed COVID-19 patients
Table 7 and Fig. 6 show the efficacy of colchicine on mortality among PCR confirmed COVID-19 Patients. Colchicine did not show a significant effect on mortality among PCR confirmed COVID-19 patients (RR 1.02, 95% CI 0.74–1.41).
Mortality among PCR confirmed COVID-19 patients
See Fig. 6.
Hospitalization among PCR confirmed COVID-19 patients
Tardif et al. assessed the efficacy of colchicine on hospitalization and reported that colchicine resulted in decreased hospitalization among the PCR confirmed COVID-19 patients (RR 0.75, 95%CI 0.57–0.99, P 0.042) [23].
Mechanical ventilation among PCR confirmed COVID-19 patients
Tardif et al. found that colchicine has no significant effect on mechanical ventilation among PCR confirmed COVID-19 Patients (RR 0.50, 95%CI 0.23–1.07, P 0.042) [23].
Discussion
In this meta-analysis, the studies investigated the role of colchicine in the management of COVID-19 were reviewed.
After a comprehensive search, eight studies were identified. Two of them were cohort studies (Brunetti et al., and Scarsi et al.) while the other studies were four randomized control trials (Deftereos et al., Recovery Collaborative Group, Lopes et al., and Tardif et al.) and two non-randomized trials (Mareev et al., and Sandhu et al.). The current meta-analysis involved 16,488 patients; 8146 were in the treatment group who received colchicine and 8342 were in the control group who received a placebo or standard treatment [20,21,22,23,24,25,26,27].
The efficacy of colchicine on mortality
The eight pooled studies evaluated the efficacy of colchicine on mortality among COVID-19 patients and showed a significant reduction in the mortality rate among patients received colchicine in comparison with placebo or standard care. This result coincides with the findings of a recent systematic review that reported a significant decrease in the all-cause mortality in three observational studies [28]. In addition, a recently published meta-analysis reported that colchicine resulted in decreased mortality among COVID-19 patients. This study pooled four randomized control trials and five observational studies and involved 5522 patients only [29].
On the other hand, Mehta, et al. and Toro-Huamanchumo, et al. documented that colchicine had no effect on the mortality rate among COVID-19 patients [30, 31].
The heterogeneity test between the pooled studies showed a significant difference, which indicates interstudy variation. Pooling of these heterogeneous studies added more useful information.
According to our result, colchicine may have a beneficial effect to decrease mortality among COVID-19 patients. It was obvious that this effect occurred when colchicine was used within the early days of the disease. These findings can be explained by the anti-inflammatory role of colchicine that is mediated through the interaction between colchicine and microtubules which play an important role in cellular division, migration, and adhesion. This effect robustly influences the immune system response and reduces the inflammatory reaction. Also, colchicine decreases the release of cytokines and inflammatory mediators that stimulate the immune cells [32].
The subgroup analysis of the two cohort studies demonstrated a significant effect of colchicine on mortality among COVID-19 patients. However, the subgroup analysis for the six clinical trials showed that colchicine has no effect on mortality in the management of COVID-19. This result is consistent with the pooled analysis of a recent study where four clinical trials only were included [33]. This variation could be attributed to difference of the study design, variation in follow up duration and the colchicine regimen used in these studies.
The efficacy of colchicine on the duration of COVID-19 illness till recovery
The efficacy of colchicine on the duration of COVID-19 illness was assessed in three clinical trials. Lopes et al. found that hospitalized COVID-19 patients who received colchicine had a shorter duration of illness till recovery in comparison with the patients who received placebo [23]. This is similar to the result reported by a recent study [34]. This finding can be related to the anti-inflammatory and immune modulatory roles of colchicine in the management of COVID-19. On the other hand, two clinical trials reported that colchicine did not affect the duration of COVID-19 illness [23, 25]. These findings agree with the results of a recently published study investigated the efficacy of colchicine on the duration of COVID-19 clinical course [31].
The efficacy of colchicine on need for hospitalization
Tardif et al., investigated the efficacy of colchicine among non-hospitalized COVID-19 patients vs placebo. They found that colchicine did not influence the need for hospitalization among the non-hospitalized patients [21]. A recent clinical trial was conducted to assess the effect of colchicine on the prognosis of non-hospitalized COVID-19 patients and the results showed no significant effect of colchicine on hospitalization rate of the patients [35].
The efficacy of colchicine on need for O2 therapy
Lopes et al., assessed the efficacy of colchicine on the need for O2 therapy and the results demonstrated that colchicine use resulted in a significant decrease in the need for O2 therapy in patients with COVID-19 [23]. This result can be understood based on the beneficial effect of colchicine on the inflammatory response.
The efficacy of colchicine on need for ICU admission
The pooled results of two clinical trials showed that colchicine did not improve the need of ICU admission compared to placebo or standard care. This finding is concomitant with a recent study that included six studies only [30].
The efficacy of colchicine on need for artificial ventilation
Four pooled studies evaluated the efficacy of colchicine on need for artificial ventilation and showed that colchicine did not decrease the need for artificial ventilation compared to placebo or standard care [20,21,22, 24].
The heterogeneity test between the pooled studies regarding the need for artificial ventilation showed a significant difference, which indicates interstudy variation.
This can be attributed to the variation of duration and dose of colchicine regimens in these studies, and the severity of the disease. Tardif et al., included non-hospitalized COVID-19 patients while the other three studies involved hospitalized patients.
The efficacy of colchicine on hospital discharge rate
Three pooled studies evaluated the efficacy of colchicine on hospital discharge rate and showed that colchicine did not improve the hospital discharge rate in comparison with placebo or standard treatment [22, 24, 26].
Furthermore, the subgroup analysis of the pooled results included two clinical trials and showed that colchicine did not cause a significant improvement in the hospital discharge rate compared to placebo or standard treatment [22, 24]. On the other hand, the cohort study demonstrated a beneficial effect of colchicine on the hospital discharge rate compared to standard care [26].
The variation of the results of the three studies could be attributed to the difference of study design, number of included patients, and the treatment regimens used.
Subgroup analysis among PCR confirmed COVID-19 patients
Two pooled studies evaluated the efficacy of colchicine among PCR confirmed COVID-19 patients and showed that colchicine did not significantly decrease mortality among PCR confirmed patients [21, 22].
In addition, Tardif et al. assessed the efficacy of colchicine on hospitalization rate among PCR confirmed COVID-19 patients and found that colchicine significantly decreased the hospitalization rate compared to placebo. Also, Tardif et al. evaluated the effectiveness of colchicine on mechanical ventilation rate among PCR confirmed COVID-19 patients and showed no beneficial effect of colchicine on mechanical ventilation in comparison with placebo [21].
Conclusion
The study demonstrates that colchicine administration leads to a notable reduction in mortality rates and a decrease in the necessity for oxygen therapy among individuals with COVID-19. Although its impact on broader outcomes like hospitalization rates, ICU admissions, and discharge rates remains minimal, there’s a significant finding regarding its efficacy in lowering hospitalizations specifically among PCR-confirmed COVID-19 patients. This detailed understanding highlights the potential of colchicine as a therapeutic intervention for COVID-19, particularly in mitigating mortality risks and oxygen therapy requirements. These results offer valuable insights for clinicians, highlighting the need to consider colchicine as a viable treatment option for COVID-19 patients, while also emphasizing the necessity for further exploration to optimize its clinical utility.
Availability of data and materials
Our study is a Systematic Review/Meta-analysis. The datasets analyzed during the current study are available in the published pooled study. Also, the datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Rahman MT, et al. Early prediction and HRCT evaluation of post covid-19 related lung fibrosis. Microbiol Insights. 2023;16:11786361231190334.
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–97.
Han Q, Lin Q, Jin S, You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J Infect. 2020;80(4):373–7.
Hageman JR. The coronavirus disease 2019 (COVID-19). Pediatr Ann. 2020;49(3):e99–e100.
WHO. World Health Organization. Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. 2022. Available from: https://covid19.who.int/info/.
Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, Hooft L, Emperador D, Domen J, Tans A, Janssens S, Wickramasinghe D, Lannoy V, Horn SRA, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2022;5(5):CD013665. https://0-doi-org.brum.beds.ac.uk/10.1002/14651858.CD013665.pub3.
Mehraeen E, Salehi MA, Behnezhad F, Moghaddam HR, SeyedAlinaghi S. Transmission modes of COVID-19: a systematic review. Infect Disord Drug Targets. 2021;21(6):e170721187995.
van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–7.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Pandit R, Matthews QL. A SARS-CoV-2: companion animal transmission and variants classification. Pathogens. 2023;12(6):775.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7.
Bhattacharyya B, Panda D, Gupta S, et al. Anti-mitotic activity of colchicine and the structural basis for its interaction withTubulin. Med Res Rev. 2007;28(1):155–83.
Cronstein BN, Esserman PR, Sunkureddi P. Mechanistic aspects of inflammation and clinical Management of Inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19(1):19–29.
Korkmaz S, Erturan I, NazIroǧlu M, et al. Colchicine modulates oxidative stress in serum and neutrophil of patients with Behçet disease through regulation of ca 2+ release and antioxidant system. J Membr Biol. 2011;244(3):113–20.
Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, Sen S, Duman S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(5):53-57.
Lho Y, Do JY, Heo JY, Kim AY, Kim SW, Kang SH. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. Int J Mol Sci. 2021;22(9):4739.
Schlesinger, N., Firestein, B. L., & Brunetti, L. Colchicine in COVID-19: an old drug, New Use In Current Pharmacology Reports 6(4): 137–145 (2020).
Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6)
Tardif JC, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924–32.
Group, R. C. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9(12):1419–26.
Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1):1–8.
Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection. Can J Infect Dis Med Microbiol. 2020;2020:1–9.
Mareev VY, Orlova YA, Plisyk AG, et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiya. 2021;61(2):15–27.
Brunetti L, Diawara O, Tsai A, et al. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J Clin Med. 2020;9(9):1–12.
Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-Centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–9.
Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med. 2022;54(1):775–89.
Elshafei MN, El-Bardissy A, Khalil A, et al. Colchicine use might be associated with lower mortality in COVID-19 patients: a meta-analysis. Eur J Clin Investig. 2021;51(9):1–5.
Mehta KG, Patel T, Chavda PD, et al. Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials. RMD Open. 2021;7(3):1–10.
Toro-Huamanchumo CJ, Benites-Meza JK, Mamani-García CS, et al. Efficacy of colchicine in the treatment of COVID-19 patients: a systematic review and Meta-analysis. J Clin Med. 2022;11(9)
Hariyanto TI, Halim DA, Jodhinata C, et al. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–30.
Zein AFMZ, Raffaello WM. Effect of colchicine on mortality in patients with COVID-19 – a systematic review and meta-analysis. Diabet Metabol Syndrome: Clin Res Rev. 2022;16(2):102395.
Kow CS, Lee LH, Ramachandram DS, et al. The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: a meta-analysis of randomized trials. Immun Inflamm Disease. 2022;10(2):255–64.
Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022;19(22):1–9.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Kholoud Elshiwy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Ghada Essam El-Din Amin: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Nazmy: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Rasha Samir: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft. Mohamed Farouk Allam: Field work supervision, analysis strategy and design, data management, data analysis and interpretation of results, decision making on content and paper write-up and revision of final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elshiwy, K., Amin, G.E.ED., Farres, M.N. et al. The role of colchicine in the management of COVID-19: a Meta-analysis. BMC Pulm Med 24, 190 (2024). https://0-doi-org.brum.beds.ac.uk/10.1186/s12890-024-03001-0
Received:
Accepted:
Published:
DOI: https://0-doi-org.brum.beds.ac.uk/10.1186/s12890-024-03001-0